Abstract
Background: HLH is a systemic condition characterized by inflammatory storm and immune-mediated organ damage. Malignancy-associated HLH (M-HLH) in adults is particularly challenging and highly lethal due to under recognition and diverse clinical presentation, with HLH features often obscured by the underlying cancer manifestations (Daver N, Cancer 2017; Wang N, Oncotarget 2017). A standard treatment approach has not been defined for M-HLH in adults.
Methods: We retrospectively reviewed the records of pts aged ≥ 18 years with an underlying malignancy who fulfilled ≥5 HLH-2004 criteria and were diagnosed between 2013 and 2017.
Results: 34 pts met the criteria for HLH. The median age was 59 years (range, 19-84) and 24 (71%) were men. The median time from cancer diagnosis to HLH was 8 months (mo) (range 0-177) with HLH diagnosed prior to the diagnosis of malignancy in 2 (5%) pts, concurrently at the time of malignancy diagnosis in 6 (18%) pts, during the course of treatment of the malignancy in 23 (68%) pts, and after the malignancy was in remission in 3 (9%) pts. 23 pts (68%) had AML/MDS, 8 (24%) lymphoma (B-cell, n=3; T-cell, n=5), 2 (6%) B-acute lymphoblastic leukemia, and 1 (2%) had renal cell carcinoma (RCC) treated with a PD-1 inhibitor. Among the 23 pts with AML/MDS, 17 (74%) were receiving hypomethylating agent (HMA) based therapy: HMA alone (n=13), HMA+PD-1 inhibitor (n=3,) and HMA+FLT-3 inhibitor (n=1).
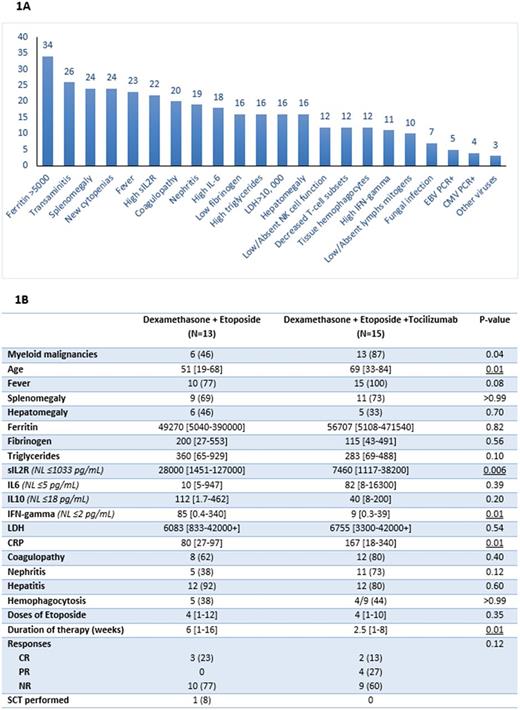
The median ferritin level was 58800 (range, 5040-471540); 29 (85%) had ferritin > 10000 and 18 (53%) had ferritin >50000. 22 (65%) had elevated sIL2R (median 9200; range, 3920-127000) (Fig 1A). Tissue hemophagocytosis was detected in 12/14 evaluated pts (86%): in the bone marrow (n=10), and/or spleen (n=2) and/or liver (n=2). Other features at HLH diagnosis were as follows: 31 pts (91%) had fever, 24 (71%) had splenomegaly, 24 (71%) had new onset ≥ 2 cytopenias, 21 (62%) had high triglycerides (≥265 mg/dL), and 16 (47%) had low fibrinogen (<150 mg/dL). Natural killer cell activity was available for 12 (35%) pts and all had low-absent function. The median lactate dehydrogenase (LDH) was 6430 (range, 1000-42000+): 30 (88%) had LDH > 2.5xULN and 16 (47%) had levels ≥10000. Clinical hepatitis, coagulopathy and nephritis were seen in 26 (76%), 20 (59%) and 19 (56%) pts, respectively. (Fig 1A). Molecular testing for genes involved in familial HLH was available for 16 pts, of whom 3 (19%) had mutations BIRC4 mutation (n=1), STXBP2 (n=1) and compound heterozygosity for PRF1 (n=1). Overall, 26 pts had a detailed interleukin (IL) panel performed at diagnosis, including IL1, IL2, IL6, IL10, IL17, IFN-γ and TNF-α, details of which will be reported later.
All 34 pts received HLH-directed therapy for a median of 2.3 weeks (range, 1-16): 6 (18%) with dexamethasone (D) alone at 10 mg/m2 twice per day (median 1 week; range 1-2). 13 (38%) pts received (D) with etoposide (E) with a median of 4 (range, 1-12) doses of etoposide. 15 (44%) pts received D+E and tocilizumab (T) with a median of 4 (range, 1-10) doses of etoposide and a median of 1.5 (range, 1-4) doses of (T). Details of pts' treatments are in Fig 1B. All pts received supportive care with broad-spectrum antimicrobials, intrathecal chemotherapy (n=18; 53%), renal replacement therapy and polyvalent immunoglobulins (when indicated).
Overall, 9 (27%) pts responded: 3 of 13 (23%) in D+E group (all had complete response (CR) of whom 1 pt proceeded to allogeneic stem cell transplantation (ASCT)) and 6 of 18 (33%) in D+E+T group (2 CR and 4 partial response (PR)). There was a trend to improved response with D+E+T versus D+E (P=0.12). At a median follow-up of 1.7 mo (range, 0.2-23.5), the median overall survival (OS) for all pts was 1.6 mo (range, 0.2-24).
Conclusion : M-HLH has poor outcomes with median OS of < 2 months in spite of therapy. Increased ferritin, tissue hemophagocytosis, fever, splenomegaly, new cytopenias, high triglycerides and LDH are frequent in adult M-HLH and presence of these features should precipitate a work-up for HLH. The addition of tocilizumab to etoposide and dexamethasone improves the response rate in patients with M-HLH but the median OS remained dismal. The presence of an active underlying malignancy, advanced age, co-morbidities and poor functional status often precluded ASCT in this population, likely contributing to dismal OS. Evaluation of novel therapies including IFN-gamma inhibitors and early evaluation and transition to ASCT may improve the outcomes in adult M-HLH.
Jabbour: Bristol-Myers Squibb: Consultancy. DiNardo: Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding. Jain: Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Celgene: Research Funding; Verastem: Research Funding; BMS: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Takahashi: Symbio Pharmaceuticals: Consultancy. Pemmaraju: Incyte Corporation: Consultancy, Honoraria; novartis: Consultancy, Honoraria, Research Funding; roche diagnostics: Consultancy, Honoraria; abbvie: Research Funding; cellectis: Research Funding; affymetrix: Research Funding; stemline: Consultancy, Honoraria, Research Funding; LFB: Consultancy, Honoraria. Wierda: Gilead: Consultancy, Honoraria, Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Karyopharm: Research Funding; Juno: Research Funding; Kite: Research Funding; Emergent: Consultancy, Honoraria, Research Funding; Acerta: Research Funding; Janssen: Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria; Merck: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; The University of Texas MD Anderson Cancer Center: Employment; Celgene: Consultancy, Honoraria. Cortes: Teva: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Sun Pharma: Research Funding; ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Kantarjian: Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Delta-Fly Pharma: Research Funding; Amgen: Research Funding. Daver: Incyte Corporation: Honoraria, Research Funding; Pfizer Inc.: Consultancy, Research Funding; Immunogen: Research Funding; Otsuka America Pharmaceutical, Inc.: Consultancy; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Kiromic: Research Funding; Daiichi-Sankyo: Research Funding; Bristol-Myers Squibb Company: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Jazz: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal